Topic:
Blood vessels are the only system to provide nutrients and oxygen to every part of the body. Many diseases can have significant effects on blood vessel formation, so the vascular network can be a cue to assess malicious tumor and ischemic tissues. Various imaging techniques can visualize the blood vessel structure, but their applications are often constrained by either expensive costs, contrast agents, ionizing radiations, or a combination of the above.
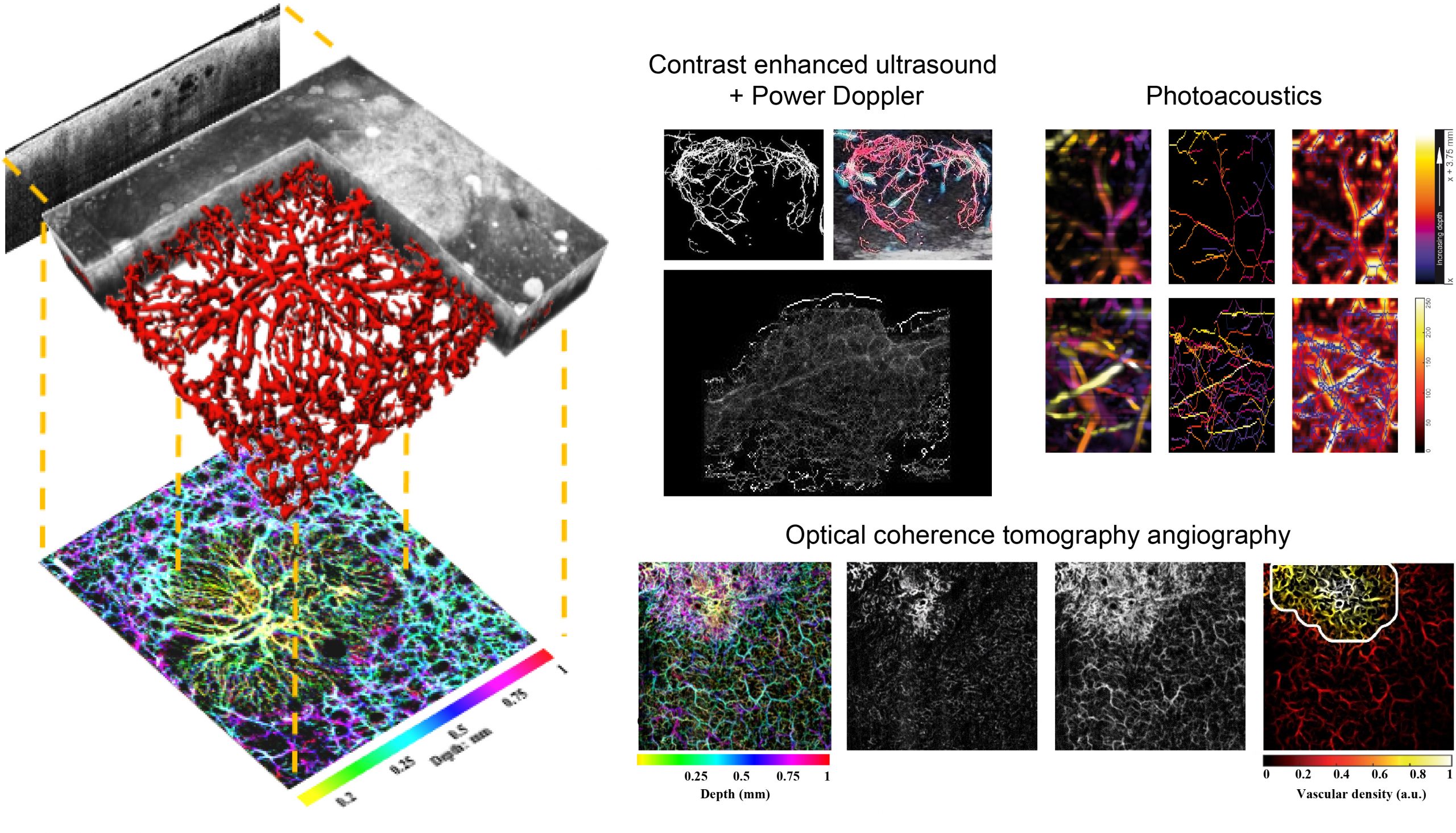
To achieve images of the vasculature using exogenous contrast agents, contrast-enhanced ultrasound (CEUS) can be employed. Microbubbles are injected and flow throughout the blood stream; using a volumetric ultrasound probe and harmonic imaging it is then possible to acquire images that show mainly where the microbubbles are located, hence the vasculature.
To achieve completely non-invasive vasculature imaging using endogenous contrast, Power Doppler ultrasound imaging can be employed, although it is limited in resolution and noisy. Another very viable option is biomedical optical imaging. Using scattering contrast, optical coherence tomography angiography (OCTA) can detect blood vessels, enabling non-invasive, in vivo, high-resolution, cross-sectional imaging in biological tissues, with a penetration depth limit to ~1–2 mm. Photoacoustic (PA) imaging combines the high-contrast and spectroscopic-based specificity of optical imaging with the high spatial resolution of ultrasound imaging. PA imaging operates in the scattering regime, but its resolution is not limited by scattering and the image contrast depends on optical absorption, making it capable of mapping blood vessels that are situated deeper in biological tissues, while still maintaining its non-invasive quality.
From the acquired volumes of these imaging modalities, a 3D map of the vasculature can be qualitatively appreciated and, more importantly, quantitative parameters can be extracted to assess the complexity of the vascular network. To do so, segmentation and skeletonization techniques are applied to the volumes and morphological and tortuosity parameters can be computed, such as the number of vascular trees, number of branches, vascular density, distance metric, inflection count metric and sum of angles metric. These parameters have shown to be able to quantitatively characterize the vascular network which can assess microenvironmental changes related to disease progression, and have been studied in our research group particularly in terms of clinical imaging of skin cancers (OCTA) and thyroid nodules (CEUS+Power Doppler), and preclinical tumor (CEUS) and burn wound healing (PA) assessment over time.
People:
Collaborators:
Funded project:
Recent publications:
Kristen M. Meiburger, Massimo Salvi, Giulia Rotunno, Wolfgang Drexler and Mengyang Liu
Applied Sciences
10.3390/app11209734
Kristen M. Meiburger, Zhe Chen, Christoph Sinz, Erich Hoover, Michael Minneman, Jason Ensher, Harald Kittler, Rainer A. Leitgeb, Wolfgang Drexler, Mengyang Liu
Journal of Biophotonics
10.1002/jbio.201900131