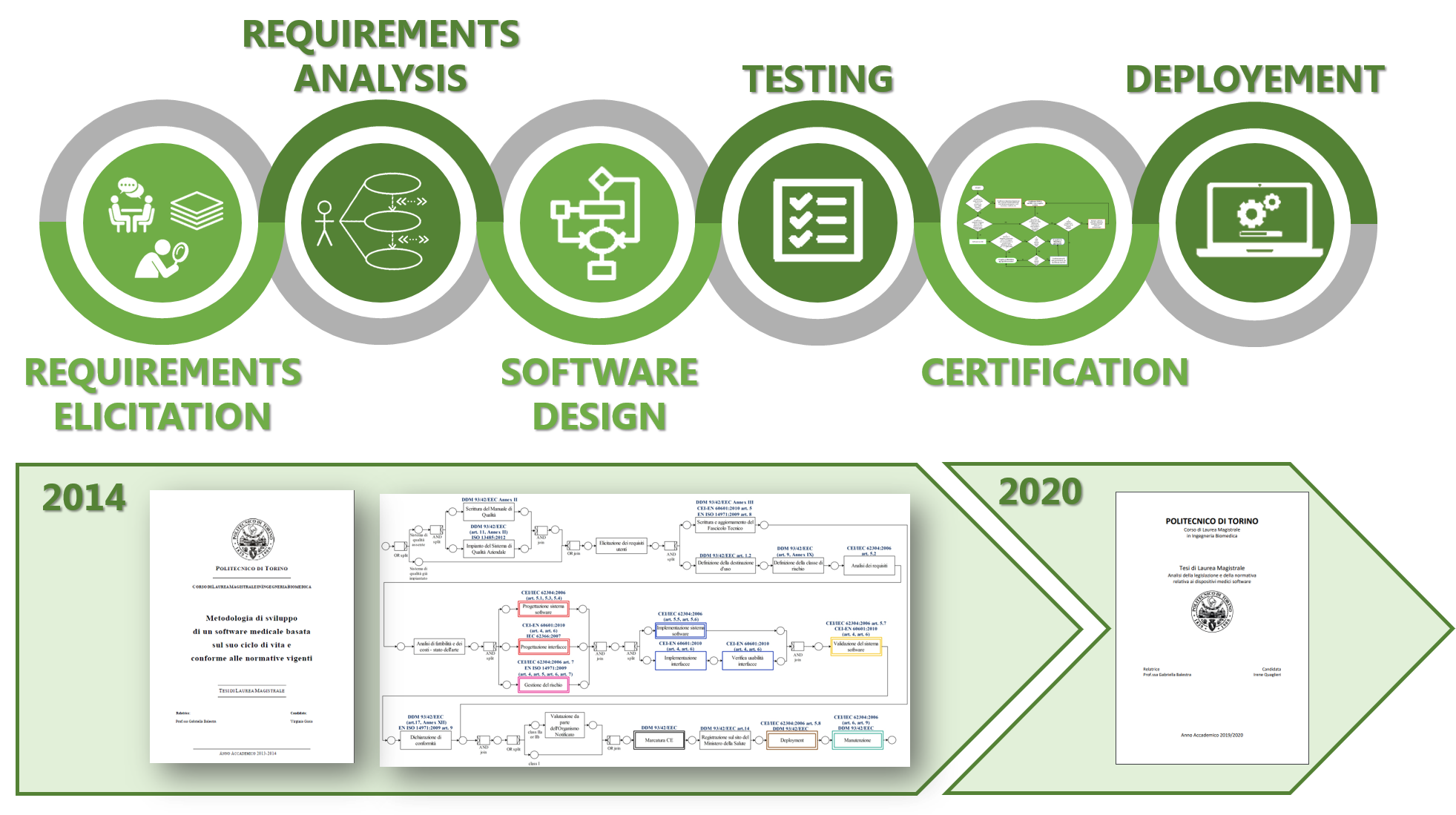
Topic:
Medical device software is playing an increasingly important role in healthcare. Designing and developing high-quality, safe, effective, usable, and innovative medical device software is a difficult task, which comprises many activities. In addition, developing safe and reliable medical device software is subjected to certification and regulatory testing. Motivated by the need to avoid risks for patients, clinicians, and people involved in the use of medical device software, regulatory agencies request that special attention be paid to all processes followed during both development and maintenance.
We support the development of new medical device software in all its phases: the requirements elicitation by means of process modeling tools to deeply understand users’ needs; the requirements analysis, the software design and testing using UML standard, the certification according to the Regulation (EU) 2017/745 of the European Union (EU) to obtain the CE mark, and finally the software deployment.